







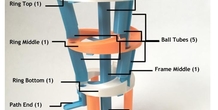
Description
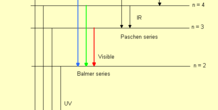
This is a model of the electron transitions that occur in atoms, but represented in terms of a hydrogen atom.
In a hydrogen atom, the single electron can "jump" to higher excited energy levels, but can also jump back down to the ground level of hydrogen. Jumping up requires energy, but jumping down releases energy, in the form of light. This model demonstrates the idea of energy being absorbed and released by a hydrogen atom and shows the types of series of the transitions. The transition series demonstrated with this model are: Lyman (n to 1), Balmer (n to 2), and Paschen (n to 3).
This lesson plan was a submission in the Create to Educate lesson plan contest with MatterHackers, sponsored by Ultimaker





Comments (1)
Sign in to leave a comment.
No comments yet. Be the first to comment!